Testing the long term enzymatic degradation hydrogels using the ElastoSens™ Bio
This is a short report of a study performed by Caroline Ceccaldi, Satu Strandman, Eve Hui,
Emmanuel Montagnon, Cédric Schmitt, Anis Hadj Henni and Sophie Lerouge at École de technologie supérieure (ÉTS) (Montreal, Canada) entitled Validation and Application of a Non-Destructive and Contactless Method for Rheological Evaluation of Biomaterials and published in 2016 on the Journal of Biomedical Materials Research Part B (105B:2565–2573)
- The evaluation of hydrogels degradation through their viscoelastic properties is conventionally performed with destructive techniques.
- ElastoSens™ Bio was used to test non-destructively the viscoelasticity of degrading hydrogel samples over 7 days.
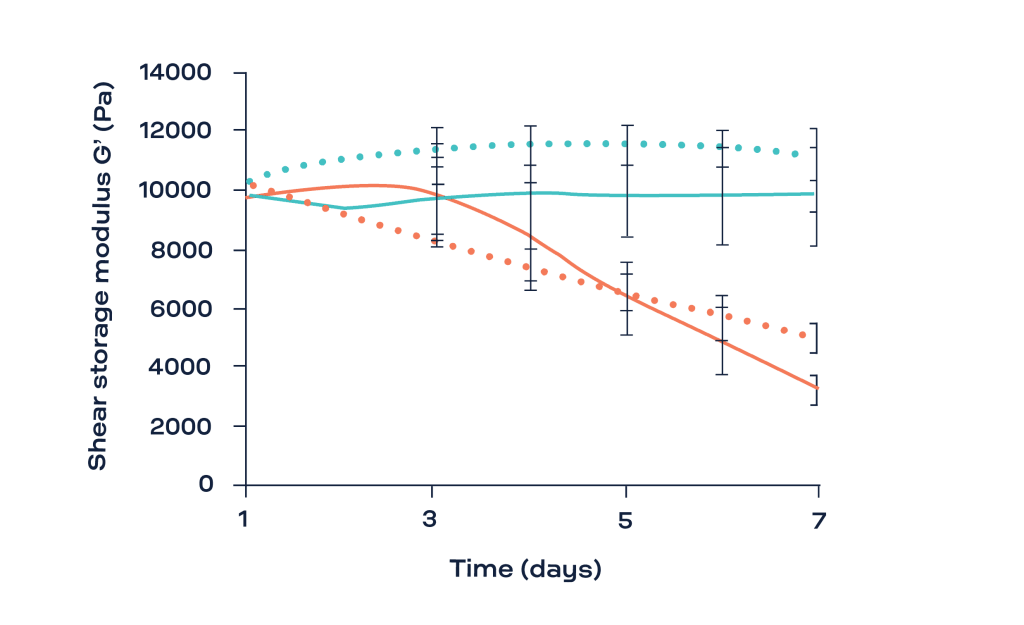
- ElastoSens™ Bio has shown to provide long-term, reproducible and sensitive measurements of the degradation of chitosan hydrogels under simulated physiological conditions.
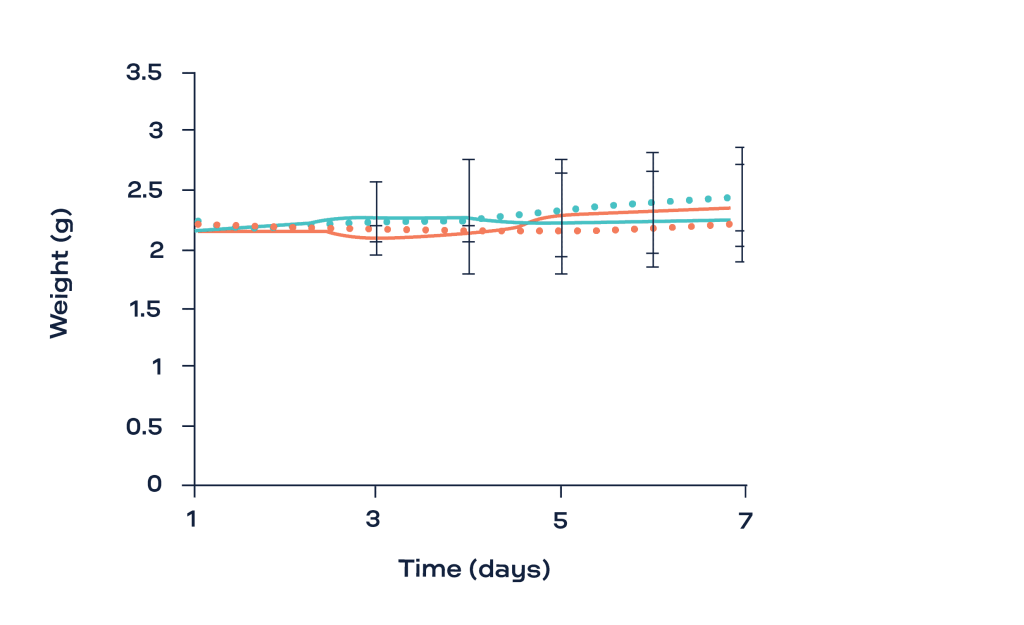
- The decrease in the shear storage modulus of chitosan hydrogels immersed with a lysozyme solution supported their degradation even without any significant change in their weight.
INTRODUCTION
Hydrogels exhibit a pronounced viscoelastic behavior similar to soft tissues. For this reason, they have been widely used in biomedical research for developing engineered tissues and novel treatments such as wound dressings and drug delivery systems. In this field, hydrogels can be composed of biodegradable natural (e.g. collagen, fibrin, chitosan) and/or synthetic polymers (e.g. PLA, PGA, PLGA). Natural materials can be degraded by naturally occurring enzymes which is an important feature for releasing a drug entrapped in the hydrogel matrix [1] or for allowing the formation of new tissues by seeded cells [2]. Therefore, the precise evaluation of this process is important for designing biomatrices with controlled degradation rates or simply for assessing whether degradation is within the desirable range for the application. In literature, degradation processes have been evaluated by the weight loss of a sample or by its mechanical properties. Since the initial stages of degradation can happen without any loss of weight, the mechanical properties can provide more precise information of the whole degradation process. However, conventional testing technologies such as rheometers and compression mechanical testers are destructive and prevent the re-use of samples for further characterizations (multiple samples are required to study degradation over long periods of time). In this short application note, chitosan-based hydrogels were prepared and immersed with lysozyme solution for the evaluation of their degradation through their viscoelastic properties using ElastoSens™ Bio. The same sample was tested each day during a week in contact with the enzyme.
MATERIALS AND METHODS
Two different sources of chitosan: (1) HDDA MMw: Chitosan with high degree of deacetylation (DDA) and medium molecular weight (ref PSN 326–501, MarinardBiotech, Gaspe, Canada), and (2) LDDA MMw: Chitosan with low DDA and medium molecular weight (ref 448877, Sigma, St. Louis, USA) were prepared by solubilizing chitosan powder in 0.1 M hydrochloric acid (3.33 % w/v). Chitosan and gelling agents were mixed at a volume ratio of 3:2, respectively. After rapid mixing, the solution (2 mL) was poured into the ElastoSens™ Bio sample holder, which was then kept at 37 °C with PBS for 24 hours. PBS was removed and the gel was tested in the ElastoSens™ Bio to obtain their initial G’ atday 1. After this time point, the samples were divided into two groups, which received either PBS (control groups) or lysozyme (human lysozyme, Sigma, 1mg/ml in PBS). The solutions were added on top of the samples inside the sample holders and they were incubated at 37 °C for 7 days. The viscoelastic tests were performed every day using the same samples during the whole study. Simultaneously, the weight of the samples was measured using an analytical balance (Mettler Toledo, model ML104, Toronto, Canada), with a readout accuracy of 0.0001 g.
RESULTS AND DISCUSSION
Fig. 1 shows the evolution of the shear storage modulus (G’, Pa) as a function of time for the two chitosan hydrogels (HDDA and LDDA) with and without the digestion solution (lysozyme). The G’ was stable for the samples immersed in PBS over the 7 days of the study. The progressive decrease in the G’ of the hydrogels after being immersed with the lysozyme reflects their degradation. Interestingly, the weight of all samples was stable during the whole experiment. This was explained by the fact that at the initial stage of degradation by hydrolysis, these physical hydrogels can keep their cohesiveness and hydrophilicity despite the decrease in chain interactions [3].
CONCLUSION
PERSPECTIVES
- ElastoSens™ Bio provides relevant information to complement the standard weight test used to study degradation.
- ElastoSens™ Bio is able to capture subtle mechanical changes during degradation before the sample starts to disintegrate.
- Testing the same sample over time is now possible due to the non destructive nature of ElastoSens™ Bio.
- Viscoelasticity testing can be performed under simulated physiological and sterile conditions.
ElastoSens™ Bio
ElastoSens™ Bio
REFERENCES
Related Posts
Enzymes are commonly used in the field of regenerative medicine to degrade native tissues for the extraction of cells and other components, or as a part of physiological-like fluids to mimic in vivo conditions for biomaterials in development. The performance of enzymes can be measured in terms of weight loss or mechanical properties. The viscoelastic properties of kidney tissue during incubation with collagenase was precisely measured using the ElastoSens™ Bio...
Cellularized hydrogels have been widely investigated for producing in vitro models of tissues such as skin, blood vessels, bone, etc. These models can be a valuable alternative to animal models used in trials for studying physio/pathological processes and for testing new drugs and medical devices.
The thermoreversible behavior of some polymers relies on the large conformation changes in response to temperature. They have been investigated for a variety of clinical applications that demand an in situ gelation at physiological temperatures. In addition, these polymers have been widely studied for other biomedical applications such as drug delivery and tissue engineering in which the thermoresponsive behavior needs to be balanced with biocompatibility and degradation kinetics.
The controlled release of drugs at precise locations within the body can prevent systemic toxicity and deliver accurate dosages to patients. Hydrogels have recently been investigated as promising drug delivery systems due to their ability to provide spatial and temporal control over the release of a number of therapeutic agents. Furthermore, the easy tunability of their physicochemical and mechanical properties allows the design of application-specific release systems.
Biodegradable hydrogels are promising candidates as drug carriers due to their biocompatibility and tunable degradation. This is particularly valuable for oral delivery systems since the polymer should respond to pH or enzymatic changes in the gastrointestinal environment to achieve a controlled drug release.







