Degradation and drug release of hydrogel-based drug delivery systems
SUMMARY
- One of the release mechanisms of drug delivery systems based on hydrogel involves the degradation of the matrix that carries the drug.
- Conventional testing instruments are difficult to use for evaluating the mechanical properties of hydrogel-based drug release systems during degradation.
- ElastoSens™ Bio has shown to provide precise measurements of the mechanical properties of dye-loaded gelatin/alginate composite gels.
- The temperature-dependent degradation of the gelatin beads promoted the dye release from the composite gels.
INTRODUCTION
The controlled release of drugs at precise locations within the body can prevent systemic toxicity and deliver accurate dosages to patients. Hydrogels have recently been investigated as promising drug delivery systems due to their ability to provide spatial and temporal control over the release of a number of therapeutic agents. Furthermore, the easy tunability of their physicochemical and mechanical properties allows the design of application-specific release systems [1]. One of the release mechanisms of therapeutic agents is based on the controlled degradation of the hydrogel that carries the drug. In this case, the decrease in the viscoelastic properties of the hydrogel is proportional to the amount of released drug [2]. Because conventional mechanical testing techniques are destructive, they prevent the re-testing of the same hydrogel sample for long term degradation studies. The use of these instruments requires the monitoring of multiple samples for a complete drug release study as a function of time. In addition, technical limitations of traditional instruments are usually an issue to precisely measure the soft nature of hydrogels.
In this short application note, the ElastoSens™ Bio was used to non-destructively monitor the viscoelastic properties of a degrading composite hydrogel made of dye-loaded gelatin beads and alginate. The dye was used to simulate the drug. Its release was measured along the study and correlated to the composite hydrogel viscoelasticity.
MATERIALS AND METHODS
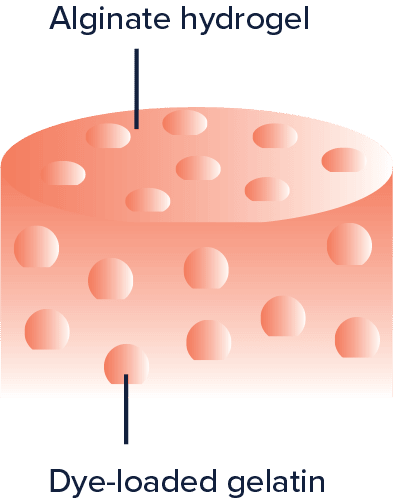
Bovine gelatin (Sigma-Aldrich, MO, USA) was dissolved in deionized hot water (10 % w/w) and combined with green food dye (Club House). The dye in this study was used to simulate the therapeutic agent added in the matrix of the drug delivery system. The dye-loaded gelatin solution was dropped into ice-cold mineral oil to form gel beads and inserted in the ElastoSens™ Bio sample holders. Alginate (5 %) and CaCl2 (crosslinking agent) solutions were added to form the matrix around the dye-loaded gelatin beads (Fig. 1). After gelling overnight, the gelatin beads were uniformly distributed in the alginate matrix and deionized water was added on top of each composite gel. The samples were incubated at 4 °C, room temperature, 32 °C and 37 °C for 24 hours each. At the end of incubation, images from the supernatant were analyzed by ImageJ to quantify the dye release and the viscoelasticity of the gel samples was tested in the ElastoSens™ Bio at the same temperature.
RESULTS AND DISCUSSION
Fig. 2 shows the effect of temperature on the shear storage modulus (G’) of the composite gels (A) and the 24h-cumulative dye release as a function of the shear storage modulus of the composite gels after the incubation at each temperature (B). The samples that were maintained for 24 hours at higher temperature showed lower shear storage moduli (G’). As expected, the cumulative amount of dye released after each 24 hours increased with the softness of the composite hydrogel (Fig. 2, B) suggesting that the diffusion of the dye was faster when the carrying gels were soft. The melting of the gelatin beads promoted the release of the dye and its diffusion to the supernatant. It was observed that all samples maintained their shape integrity even at higher temperatures.


CONCLUSION
PERSPECTIVES
- ElastoSens™ Bio is able to capture subtle mechanical changes during degradation before the sample starts to disintegrate.
- ElastoSens™ Bio can test viscoelasticity under simulated physiological conditions.
- ElastoSens™ Bio can be used to develop/formulate hydrogels, to tune their degradation profile and to test/optimize degradation mechanisms (enzymatic degradation, hydrolysis, etc.).
- Viscoelasticity measurements and degradation profiles can be correlated with the amount of drug released in order to control the release for specific therapeutic applications.
ElastoSens™ Bio
ElastoSens™ Bio
REFERENCES
[1] Li, J., & Mooney, D. J. (2016). Designing hydrogels for controlled drug delivery. Nature Reviews Materials, 1(12), 1-17.
[2] Chou, S. F., & Woodrow, K. A. (2017). Relationships between mechanical properties and drug release from electrospun fibers of PCL and PLGA blends. Journal of the mechanical behavior of biomedical materials, 65, 724-733.
Related Posts
Controlled drug delivery systems (CDDS) have been developed as a solution to overcome challenges associated with conventional drug administration routes. These systems consist of a polymeric matrix that contains the therapeutic agent and allows for controlled release of the drug over a prolonged period of time. CDDS offer advantages such as improved drug absorption, reduced side effects, and enhanced therapeutic efficacy. They can be designed for various routes of administration, including oral, transdermal, inhalation, and rectal routes. By utilizing CDDS, drug concentrations in the blood can be maintained at optimal levels for an extended duration, resulting in improved therapeutic outcomes. Researchers continue to explore and refine these systems to optimize drug delivery and enhance patient care.
Cellularized hydrogels have been widely investigated for producing in vitro models of tissues such as skin, blood vessels, bone, etc. These models can be a valuable alternative to animal models used in trials for studying physio/pathological processes and for testing new drugs and medical devices.
The thermoreversible behavior of some polymers relies on the large conformation changes in response to temperature. They have been investigated for a variety of clinical applications that demand an in situ gelation at physiological temperatures. In addition, these polymers have been widely studied for other biomedical applications such as drug delivery and tissue engineering in which the thermoresponsive behavior needs to be balanced with biocompatibility and degradation kinetics.
Biodegradable hydrogels are promising candidates as drug carriers due to their biocompatibility and tunable degradation. This is particularly valuable for oral delivery systems since the polymer should respond to pH or enzymatic changes in the gastrointestinal environment to achieve a controlled drug release.
Hydrogels exhibit a pronounced viscoelastic behavior similar to soft tissues. For this reason, they have been widely used in biomedical research for developing engineered tissues and novel treatments such as wound dressings and drug delivery systems.







