Tissue Engineering
Tissue engineering has emerged from the need for alternative grafts to auto or allo-transplantation. Engineered tissues have been developed using biomaterials and cells to mimic the properties of the damaged tissue with the ultimate purpose of repairing or replacing it.
The mechanical properties of engineered tissues evolve with time since cells are able to degrade the initial biomaterial (their scaffold) and produce ECM proteins. Collagen, gelatin, chitosan, alginate, fibrin, agarose, PEG, PLGA, PCL are among the most popular hydrogel biomaterials.
Due to their soft nature and complex preparation, measuring the viscoelasticity of engineered tissues is challenging and monitoring their changes during culture time is not often possible.

We have designed the ElastoSens™ Bio to answer these needs. The instrument tests the viscoelasticity of cellularized scaffolds by applying gentle vibrations to the sample and measuring with no contact its response to the mechanical stimulus. The cellularized scaffold is contained in a detachable sample holder and the whole system can be inserted in a biological hood for testing under sterile conditions. The sample holder containing the engineered tissue can be placed in the incubator and retested multiple times during culture time. At each time point, the instrument measures and displays the current storage (G’) and loss (G’’) shear modulus of the evolving cellularized scaffold.
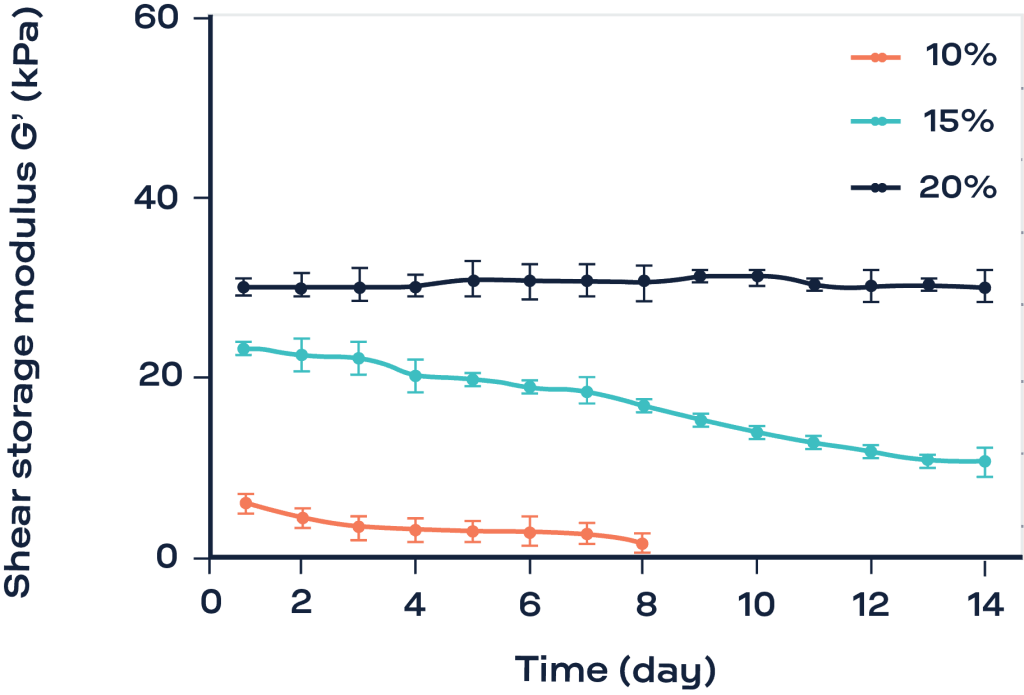
In this example, keratinocytes laden gelatin samples were tested during 14 days of culture using the ElastoSens™ Bio. The gels were prepared at concentrations of 10 %, 15 % and 20 % (w/w%) of gelatin. The higher concentration of gelatin led to a mechanically stable construct whereas a significant degradation was observed for the other two conditions.


Benefits of Contact-Free, Non-Invasive Measurements with the Elastosens™ Bio
- Test non-destructively the viscoelastic properties of bulk hydrogels, 3D bioprinted scaffolds and 3D cell-laden hydrogels.
- Apply programmable thermo and photo (UV) stimulations to deeply analyze your material.
- Follow the evolution of the same sample from formation to degradation non-destructively and over long periods of time.
- Get advanced and personalized Soft Matter Analytics™.
- Accelerate your formulation process while improving repeatability.
- Test bioengineered tissues in a cell-friendly and sterile environment.
- Operate a truly easy-to-use instrument designed for biologists, chemists and material scientists.
- Save time and material for R&D and QC operations.
- Customize your own testing system with up to 5 instruments thanks to the modularity of the ElastoSens™ Bio.
- Optimize your investments with affordable instruments that fit your needs and budget.
Visit the ElastoSens™ Bio product page
Related Application Notes
The mechanical characterization of biological soft membranes such as skin, pericardium, or hydrogel-based samples is challenging and often results in sample damage. The Membrane Sample Holder of the ElastoSens™ Bio has been designed for facilitating the loading and the handling of membrane samples allowing proper mechanical testing. In this study, the elasticity of bovine pericardium membranes were precisely measured using the ElastoSens™ Bio...
The field of regenerative medicine comprises different strategies to replace or restore diseased and damaged tissues and organs. It includes tissue-engineered products that rely on the combination of biomaterials, cells and inductive biomolecules to promote tissue and organ regeneration.
All cells in the human body are exposed to mechanical forces which regulate cell function and tissue development, and each cell type is specifically adapted to the mechanical properties of the tissue it resides in. The matrix properties of human tissues can also change with disease and in turn facilitate its progression.
Cellularized hydrogels have been widely investigated for producing in vitro models of tissues such as skin, blood vessels, bone, etc. These models can be a valuable alternative to animal models used in trials for studying physio/pathological processes and for testing new drugs and medical devices.
Related Scientific Articles
This study introduces two novel smart polymer 3D-printable interpenetrating polymer network (IPN) hydrogel biomaterials for potential applications in traumatic brain injury (TBI). These IPN biomaterials show favorable chemical, mechanical, and morphological properties and can potentially assist in the restoration of neurological function and neural tissue regeneration. The scaffolds were prepared using collagen, elastin, and gelatin methacryloyl, and were crosslinked with Irgacure or Irgacure and Genipin. The biomaterials exhibited thermal stability, amorphous nature, and maintained the peptide secondary structure. With a stiffness suitable for softer tissue engineering applications, the IPN biomaterials resemble the native rat cortex. They supported the growth of PC12 cells and showed antimicrobial properties. However, it was observed that the full IPN was more brittle than the semi IPN, which was contradictory to previous literature findings. Overall, this research contributes to the development of potential biomaterials for TBI applications and 3D printing, paving the way for patient-specific scaffolds in neural treatments.
This paper presents a new approach for estimating arterial stiffness using guided wave inversion (GWI) in shear wave elastography. The approach incorporates refinements in signal processing, an efficient forward model for computing theoretical dispersion curves, and an optimization framework based on the multimodal nature of the measured dispersion curve. The method is validated using experimental data from rubber tube phantoms, demonstrating accurate estimation of modulus and wall thickness. The approach shows less than 4% error in modulus estimation for 70% of the experiments and can be executed in less than half a minute. This new GWI approach holds promise for assessing arterial stiffness as a biomarker for cardiovascular diseases.
The development of biomaterials and engineered tissues are reshaping the future of medicine. Soft and injectable materials are increasingly used to treat pathologies, release drugs and/or seed cells for tissue engineering. Mechanical properties of a biomaterial are critical for its functional efficiency. However, the non-destructive mechanical characterization of soft biomaterials, especially in presence of cells, is still a challenge. The aim of this work was to validate the use of a new instrument, ElastoSens™ Bio2 (Rheolution Inc., Montréal, QC), that measures in real-time, nondestructively and without contact the evolution of rheological properties of viscoelastic biomaterials during reticulation kinetics and tissue culture. This study introduces this new technology and compares it with classical rheometry. The gelation kinetic of various hydrogels was studied by both techniques in parallel. The cytocompatibility of the instrument’s sample-holder was verified. Finally, preliminary results of viscoelastic measurements performed on a three-dimensional culture of fibroblasts in hydrogels during culture period are presented.
Chitosan (CH) hydrogels with remarkable mechanical properties and rapid gelation rate were recently synthesized by combining sodium hydrogen carbonate (SHC) with another weak base, such as beta-glycerophosphate (BGP). To improve their biological responses, in the present study, chondroitin sulfate (CS) was added to these CH hydrogels. Hydrogel characteristics in terms of pH and osmolarity, as well as rheological, mechanical, morphological and swelling properties, were studied in the absence and presence of CS. Effect of CS addition on cytocompatibility of hydrogels was also assessed by evaluating the viability and metabolic activity of encapsulated L929 fibroblasts. New CH hydrogels containing CS were thermosensitive and injectable with pH and osmolality close to physiological levels and enhanced swelling capacity. Encapsulated cells were able to maintain their viability and proliferative capacity up to 7 days and CS addition improved the viability of the cells, particularly in serum-free conditions. Addition of CS showed a reducing and dose-dependent effect on the mechanical strength of the hydrogels after complete gelation. This work provides evidence that CH-CS hydrogels prepared with a combination of SHC and BGP as a gelling agent have a promising potential to be used as thermosensitive, injectable and biocompatible matrices with tunable mechanical properties for cell therapy applications.
This study introduces a novel approach in fabricating bioplatforms with favourable physical, chemical, and mechanical properties for wound dressing applications. The approach employs a three-step method; partial crosslinking of polymers into soft macromatrices, lyophilization, and pulverization of those macromatrices to obtain polymer particles with improved properties. For investigation of this approach, the ionic polysaccharides, sodium alginate and chitosan were partially crosslinked with calcium chloride and sodium tripolyphosphate, respectively, followed by interpolymer complexation (IPC) for formation of the bioplatform. The formulations displayed good thermal stability with enhanced water uptake. The IPC exhibited water uptake of 4343.4% over 24 h and displayed 78% biodegradation over 14 days, which was superior to that of a commercial alginate-based wound dressing (1612.56% swelling and 16.26% biodegradation). The bioplatform thus possessed promising fluid-absorptivity and biodegradability, for potential application as a wound therapeutic system.
Wound infection is a major clinical challenge that can significantly delay the healing process, can create pain, and requires prolonged hospital stays. Pre-clinical research to evaluate new drugs normally involves animals. However, ethical concerns, cost, and the challenges associated with interspecies variation remain major obstacles. Tissue engineering enables the development of in vitro human skin models for drug testing. However, existing engineered skin models are representative of healthy human skin and its normal functions. This paper presents a functional infected epidermis model that consists of a multilayer epidermis structure formed at an air-liquid interface on a hydrogel matrix and a three-dimensionally (3D) printed vascular-like network. The function of the engineered epidermis is evaluated by the expression of the terminal differentiation marker, filaggrin, and the barrier function of the epidermis model using the electrical resistance and permeability across the epidermal layer. The results showed that the multilayer structure enhances the electrical resistance by 40% and decreased the drug permeation by 16.9% in the epidermis model compared to the monolayer cell culture on gelatin.